What are the most promising technologies for low-carbon hydrogen?

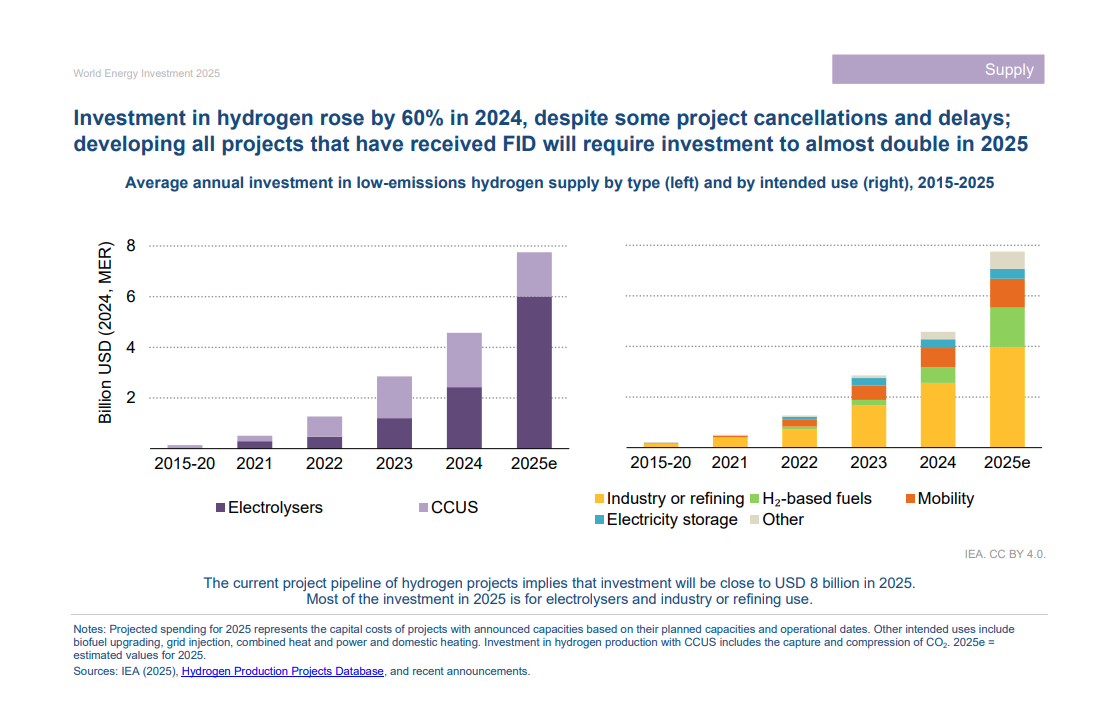
While momentum for low-carbon hydrogen has dipped as countries have prioritized energy security over sustainability, the International Energy Agency put the project pipeline for 2025 at about $8 billion, almost double 2024’s level.
Analysts PV Tech Research are forecasting a 200% annual growth in green hydrogen in Europe alone, with more than 60 projects currently under construction. Announcements like Microsoft's seven-year deal to source steel from green hydrogen for data centers suggest a potential uptick in demand from the hard-to-abate industries. At the same time, markets such as the EU are doubling down on policy frameworks [link to Joao’s article for PEI, once published] and technology is advancing.
With many sustainable production methods still at experimental or demonstration stages, what are the most promising ones to look out for?
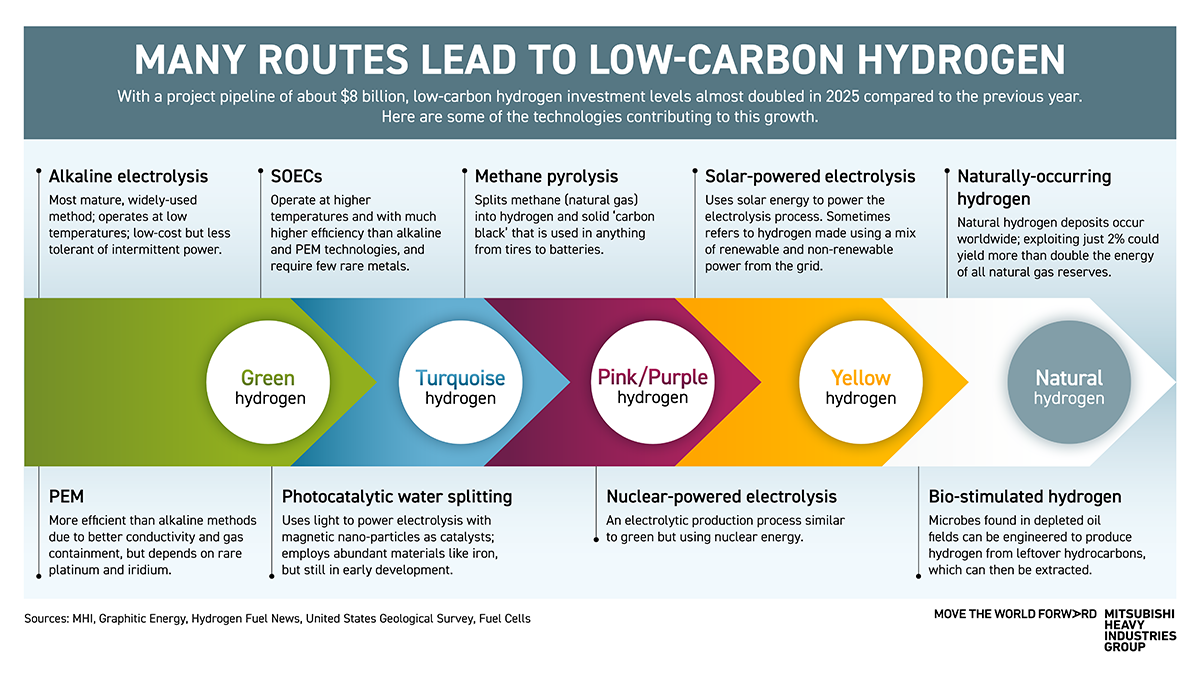
Alkaline electrolysis
Steam methane reforming – or grey hydrogen – remains the most widely used production method, accounting for 68% of global production and emitting up to 12kg of CO2 per kilogram of hydrogen produced. Water electrolysis, which is considered ‘green’ if renewable energy is used, makes up just 5%.
Among this percentage, alkaline electrolysis is the most established and commonly used. It consists of two electrodes – an anode and a cathode – with a porous separator (diaphragm) between them. An alkaline solution surrounds them. As electricity is applied, water molecules separate at the negatively charged cathode, releasing hydrogen. A low-temperature technique, it typically operates at 50-80°C. While low-cost, it is less tolerant of the power fluctuations that come with using renewable energy.

Proton exchange membrane (PEM) electrolysis
Proton exchange membrane (PEM) electrolysis uses a similar technological approach, at low temperatures, but with a polymeric membrane instead of a diaphragm. These improve conductivity and gas containment, making them more efficient than alkaline electrolyzers. PEM electrolyzers are less affected by power fluctuations than alkaline methods, but currently rely on platinum and iridium, some of the world’s rarest elements.

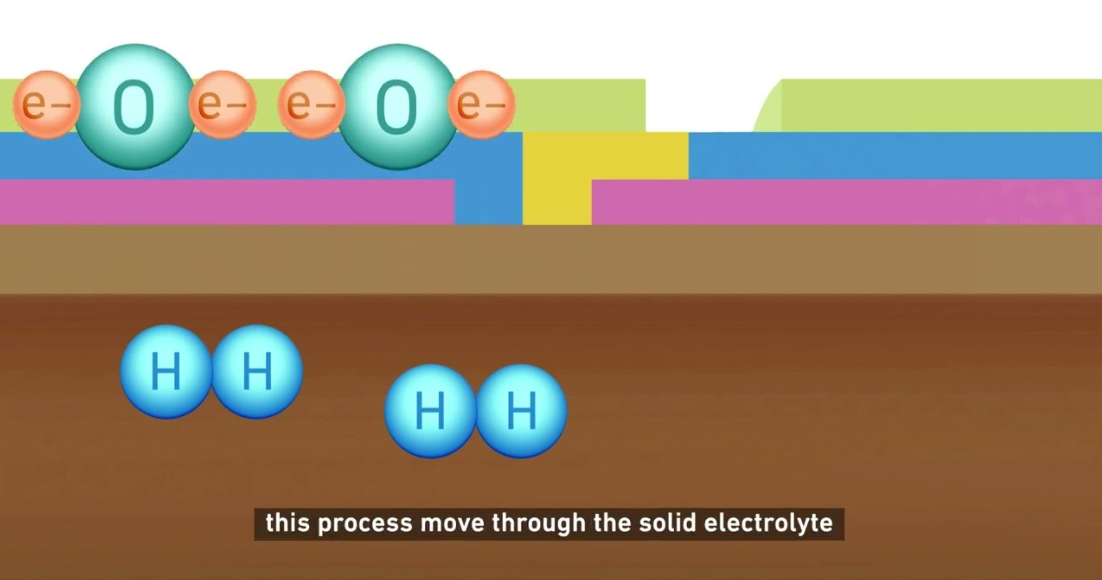
Solid-oxide electrolysis cell (SOEC)
More recently, the solid-oxide electrolysis cell (SOEC) has garnered increasing attention. It applies the same electrolytic process but requires much higher temperatures of between 600°C and 1,000°C, with ceramic cells acting as separators. Thanks to this, an SOEC can be up to 85% efficient, and Mitsubishi Heavy Industries (MHI) is targeting 90% for its own SOEC units, considerably above what alkaline and PEM methods can achieve. The SOEC derives from solid-oxide fuel cells, a mature technology that requires very few rare metals.
Photocatalytic water splitting
As the name implies, photocatalytic water splitting uses light to power the electrolysis of water with nano particles called spinel ferrites acting as the catalyst in the process. As they are magnetic, they can easily be retrieved and reused. The process uses abundant materials, such as iron, which could lead to substantial cost savings when the technology transitions from academia to real-world applications.
Methane pyrolysis
MHI is also developing methane pyrolysis to create ‘turquoise hydrogen’ by splitting natural gas into solid carbon and hydrogen. MHI is working to enhance the hydrogen production efficiency of the process. Coproduced carbon black can be used utilized as an industrial material.

Bio-stimulated hydrogen
Alongside the many chemical processes, researchers are also looking to biology for a solution — using microbes living in depleted oil fields to convert residual oil into hydrogen. A recent field trial in California successfully produced hydrogen using the process.
Naturally occurring hydrogen
Synthetic methods have dominated the production of hydrogen to date, but there is also a significant resource of natural – or white – hydrogen in the Earth’s crust. A well in Mali has been producing natural hydrogen since 2012, and substantial reserves have since been discovered elsewhere in the world, too. The US Geological Survey estimates that if just 2% of the global resource could be extracted sustainably, this would yield about 100,000 mega tonnes of hydrogen – double the energy in all natural gas reserves.
A turning point for the hydrogen industry?
Low-carbon hydrogen has entered an important phase in its evolution, with continued growth in announced projects and policy support, despite some challenges. Whether hydrogen can move closer to becoming a major decarbonization pathway will depend on more predictable and durable policy frameworks to build demand and attract investors, and the ability of technology suppliers to bring a diverse range of solutions to commercial scale.
![]()
Discover more about MHI’s work in facilitating a green hydrogen and ammonia industry





