E-methane’s potential role in reducing emissions explained

There are probably very few similarities between cooking a meal in a kitchen on Earth and preparing one on the International Space Station (ISS). There is, however, one surprising link.
A process behind a technology being used to keep astronauts alive is also key to a popular method of making e-methane — a promising fuel of the future that one day we could be using for cooking, heating and more.
The connection lies in something called the Sabatier reaction. Synthetic methane is made by combining hydrogen produced from water via electrolysis with CO₂, often using a nickel catalyst in a process invented over a century ago by French chemist Paul Sabatier. On the ISS, this process is being used to take the CO₂ exhaled by astronauts and recycle it into water needed for oxygen production.
Back on Earth, e-methane holds promise as a low-emissions fuel with near-identical chemical and physical properties to natural gas. For that reason, amid the race to decarbonize, companies and countries across the globe are exploring its use as an alternative to gas in both domestic and industrial settings.

A low-emissions alternative to natural gas?
Emitting less CO₂ than other fossil fuels, natural gas is often described as an important fuel as the world transitions to more renewable energy sources.
Because it is interchangeable with natural gas, e-methane has the potential to help decarbonize gas networks in the future without the need to retrofit existing infrastructure such as liquefied natural gas (LNG) receiving terminals, LNG tankers, gas pipelines and consumer equipment. E-methane can be stored in a variety of ways — in salt caverns, porous formations in gaseous form, and LNG storage tanks. This means it could support the integration of renewables by storing energy to meet swings in demand.
It can also help bring together future hydrogen and methane networks, with surplus hydrogen being converted into e-methane before being injected into the methane system.
An ambitious goal for e-methane
Thanks to this potential, enthusiasm for the future role of e-methane is growing globally, with projects recently being announced in Finland, the US, Australia and more.
The Japanese gas industry, meanwhile, is targeting e-methane to make up 1% of its gas supply network by 2030, and aims to increase that share to 90% by 2050.
The country’s largest gas company, Tokyo Gas, is testing e-methane synthesis in Yokohama City with researchers at Osaka University and the Japanese space agency. Part of this project is devising new processes to create e-methane that are less heat-intensive and more efficient, including an update to the Sabatier method.
Ensuring e-methane is clean
While e-methane does create CO₂ emissions when burned, it is made by recycling captured CO₂. This means the amount of CO₂ in the atmosphere does not increase in real terms, making its emissions effectively zero.
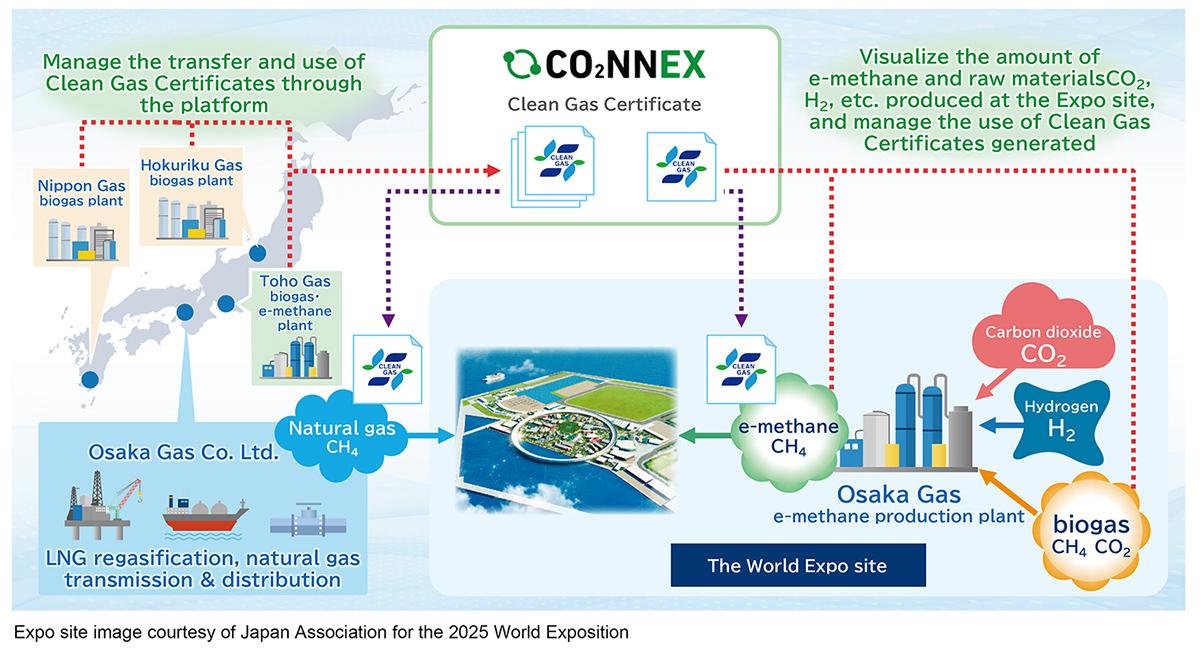
As the industry scales up, however, ensuring that e-methane has been produced with low emissions will be crucial to meeting decarbonization goals in this area. To this end, Mitsubishi Heavy Industries (MHI) Group has entered into a partnership with Japan's Osaka Gas to launch the first digital platform to manage clean gas certificates for the country’s city gas industry. The platform uses MHI's CO₂NNEX technology, which was originally developed to visualize and manage the carbon capture, utilization and storage value chain.
The digital platform from MHI and Osaka Gas will enable companies to seamlessly manage and transfer to other organizations information on e-methane, including the amount of hydrogen and CO₂ used as raw materials, the method of production and recovery, and CO₂ emissions throughout the life cycle.
The CO₂NNEX platform is being used during the 2025 World Expo in Osaka to support Osaka Gas's e-methane production and utilization demonstration. The companies intend to use the results of this trial to help support the wider application of e-methane technology across society.
Creating demand is key
Challenges remain to the uptake of e-methane. As the International Energy Agency (IEA) points out, the complex value chain supporting its production means that investment and operating costs are currently high — leaving a large gap between the cost of production and those willing to pay. Availability of feedstocks — low-emissions electricity, fresh water and renewably sourced CO₂ — is another hurdle.
Demand creation, the agency says, will be crucial to support final investment decisions. If that happens, the IEA projects that e-methane production could reach more than 1 billion cubic meters across the globe by 2030.
Sabatier’s process continues to be used by space agencies exploring the universe — and as the technology used to make e-methane evolves, it could prove to be a big step for decarbonization on Earth.
Discover more about MHI’s CO₂ capture technology





